
|
BRHS /
Dependence of burning velocity on hydrogen concentration, pressure and temperatureDahoe (DahoeAE:2005) gives an extensive comparison of the laminar burning velocities of hydrogen mixtures, obtained in (LawCK:1993), (TakahashiF:1983), (WuCK:1984), (IijimaT:1986), (DowdyDR:1990), (EgolfopoulosFN:1990), (KorollGW:1993), (VagelopoulosCM:1995), (AungKT:1997), (TseSD:2000), (KwonOC:2001), (LamoureuxN:2002). Dependence of the laminar burning velocity on equivalence ratio (H2 concentration) at normal pressure and temperatures T = 293 - 298 K , adopted from (DahoeAE:2005), is shown in Figure 1. 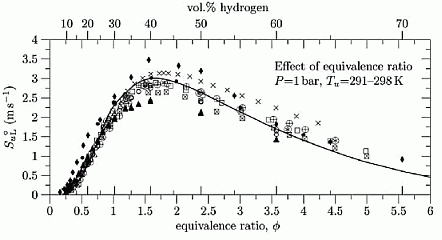 Figure 1. Effect of hydrogen concentration (expressed in equivalence ratio) on the laminar burning velocity of hydrogenair mixtures, reproduced from (DahoeAE:2005) (with permission). The H2 combustion has a unique combustion properties:
Accurate measurement of H2 burning velocity is not possible for concentrations below downward flame propagation limit 9% of H2 since anisotropic flame propagation occurs in near-limit mixtures (KorollGW:1993). Liu and MacFarlane (LiuDDS:1983) obtained burning velocities within steam concentration range 0-15%. An empirical correlation by Liu and MacFarlane developed to describe the H2-air-steam burning velocity as a function of hydrogen concentration, steam concentration and temperature, is given below: s_u = BT_u ^C \exp \left( {D\,x_{H_2 O} } \right), \ \ \ \ \ \ \ \ \ (1)
where s_u laminar burning velocity, m/s, B, C, D coefficients, T_u temperature of unburned gas in K, x - volumetric fraction of the species (H2 hydrogen, H2O - steam). Coefficients for the correlation are given in Table 1. The reported root mean square deviation of the correlation is 0.189 m/s. The authors suggested that the correlation may be applied in the whole range of hydrogen flammability limits 5-75% volume and steam concentration range 0-25% volume; experimental temperature range was 293-523K. Reported by the authors root mean square deviation of the correlation is 0.189 m/s. They suggested that the correlation may be applied in the whole range of hydrogen flammability limits 5-75% and steam concentration range 0-25%; experimental temperature range was 293-523K. 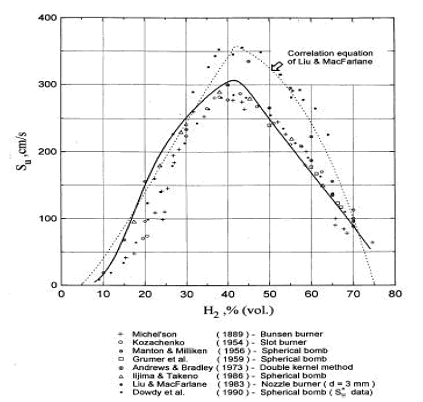 Figure 2. Laminar burning velocity of hydrogen air mixtures at room temperature and atmoshpheric measured by various methods. Solid line computed values for spherical flame propagation; dotted line correlation equation of Liu and MacFarlane. Table 1. Coefficients in the burning velocity correlation, eq. (1).
Combustion of H2-air-steam mixtures was studied later by Koroll et. al. (KorollGW:1993) within a steam concentration range 12-42% and Lamoureux et. al. (LamoureuxN:2002), who measured burning velocity with steam concentrations 10, 20, 30%. Koroll et. al. developed a correlation for burning velocity of H2-O2-diluent and H2-O2-diluent/steam mixtures, which is applicable in H2 concentration range 9% - 70%. Burning velocity of H2-O2 mixtures and hydrogen-fuelled mixtures with diluents were studied by Kwon et. al (KwonOC:1992) - H2-O2-N2 mixtures, Koroll et. al. (KorollGW:1993) - H2-air-diluent mixtures (normal pressure and temperature, hydrogen concentration range 9-70%, empirical correlation for S_u is given), Tse et. al. (TseSD:2000) - H2-O2-diluent mixtures, Kwon and Faeth [[DB, KwonOC:2001]} - H2-O2-diluent mixtures. Effect of steam and combined (CO2 + He) diluent on the burning velocity was studied in (LamoureuxN:2002) at T = 353K. Experimental data on the burning velocity at elevated pressures may be found in research by Iijima et. al. (IijimaT:1986), Kwon et. al. (AungKT:1997) 3 atm, Tse et. al. (TseSD:2000) up to 20 atm, Qin et. al. (QinX:2000) up to 5 atm, Kwon and Faeth [[DB, KwonOC:2001]} up to 3 atm, Kuznetsov et al. Invalid BibTex Entry! - up to 70 atm. Experimental data on the burning velocities at elevated temperatures are given by Liu and MacFarlane (LiuDDS:1983) in the range 295K 523K, Koroll et. al. (KorollGW:1993) at temperatures 298K and 373K, Lamoureux et.al. (LamoureuxN:2002) at temperatures 298K and 353K, Kuznetsov et. al. Invalid BibTex Entry! - at temperatures up to 573K. The cited studies used following experimental techniques. Optically accessed spherical flame propagation in a closed vessel was employed for velocity measurements in (DowdyDR:1990), (KwonOC:1992), (AungKT:1997), (TseSD:2000), (KwonOC:2001), (LamoureuxN:2002). In [DB, IijimaT:1986], (MiltonBE:1984), (DahoeAE:2005) the burning velocities were obtained using measurement in of pressure variation caused by confined deflagration in a windowless chamber. Double-kernel technique was used in (KorollGW:1993). Stabilised flames were used in (WuCK:1984) - stagnation flame and Bunsen burner, (EgolfopoulosFN:1990), (VagelopoulosCM:1995) - counterflow technique, (LiuDDS:1983) constant velocity nozzle, (QinX:2000) nozzle burner. Laminar burning velocities may be computed using fundamental kinetic and thermodynamic data. Comparison of the burning velocities predicted using different reaction mechanisms and software codes against experimental data may be found, e.g. in the papers (LiuDDS:1983), (EgolfopoulosFN:1990), (DowdyDR:1990), (VagelopoulosCM:1995), (AungKT:1997), (TseSD:2000), (QinX:2000), (KwonOC:2001), (LamoureuxN:2002), (DahoeAE:2005). 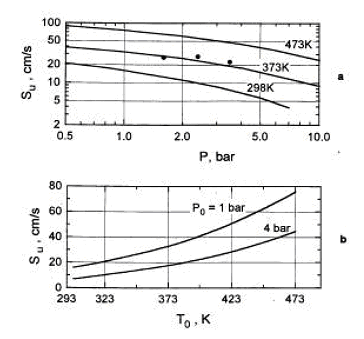 Figure 3. Laminar burning velocity as a function of pressure (a) and temperature (b) for 10% H2-air mixture (φ = 0.26.) The dependence of the laminar burning velocity on pressure and temperature is usually expressed as a polynomial function (PoinsotT:2001): s_u \left( {p,T} \right) = s_u \left( {p_0 ,T_0 } \right)\,\left( {\frac{p}{{p_0 }}} \right)^n \left( {\frac{T}{{T_0 }}} \right)^m,
where s_u \left( {p_0 ,T_0 } \right) - burning velocity at initial pressure p_0 and temperature T_0 , s_u \left( {p,T} \right) - burning velocity at actual pressure p and temperature T , m, n temperature and baric indexes in dependence of the burning velocity correspondingly. In case, where combustion process may be assumed as adiabatic (e.g., spherical flame propagation in a closed vessel) and temperature changes only due to adiabatic compression, the dependence for the burning velocity may be expressed as a function of pressure only: s_u \left( {p,T} \right) = s_u \left( {p_0 } \right)\,\left( {\frac{p}{{p_0 }}} \right)^\varepsilon ,
\varepsilon = m_0 + n_0 - m_0 /\gamma _u - overall thermokinetic index, \gamma = {{c_p } / {c_v }} - specific heat ratio, c_p, c_v - specific heats at constant pressure and constant volume correspondingly. Values of baric, thermal and overall indexes, which may be recommended according to (BabkinVS:2003), are given in Table 2. The burning velocity increases rapidly with increase of temperature and just slightly - with increase of pressure (except for mixtures close the flammability limits). Table 2. Baric, thermal and overall thermokinetic indexes for H2-air laminar burning velocity ( p_0 = 1 atm, T_0 = 298K )
* - values in brackets are interpolated or extrapolated, ** - values determined in the range p=0.25-1.0 atm. In the range of H2 concentration 60-70% values n=(-0.4) (-0.1) may be expected, (GrumerJ:1959). In paper by Iijina and Takeno (IijimaT:1986) following temperature and baric index values were reported: m = 1.54 + 0.026(\Phi - 1),
n = 0.43 + 0.003(\Phi - 1).
For stoichiometric mixture Dahoe (DahoeAE:2005) found following temperature and baric indexes using inverse problem method: m = 140.0 \pm 3.7 \cdot 10^{-2},
m = 194.0 \pm 4.4 \cdot 10^{-3}.
Dahoe A.E. (2005) Laminar burning velocities of hydrogen-air mixtures from closed vessel gas explosions. Journal of Loss Prevention in the Process Industries, 18:152-166.(BibTeX) |